Revolutionize Thyroid Treatment
Transform Thyroid Treatment with the CRF Thyroid Radiofrequency Ablation System from Cambridge Interventional
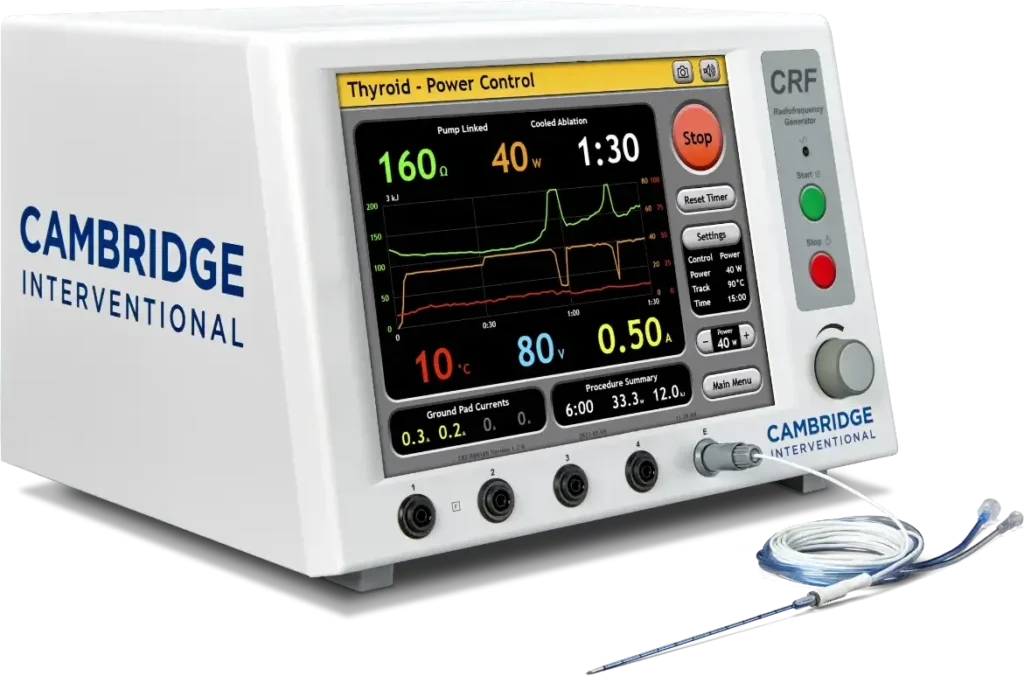
Transform Thyroid Treatment with the CRF Thyroid Radiofrequency Ablation System from Cambridge Interventional

The CRF Thyroid RFA System, exclusively from Cambridge Interventional, is the only USA-based technology for thyroid radiofrequency ablation. Designed for precision, efficiency, and patient satisfaction, it’s transforming practices like yours.
Reduces hand fatigue and improves comfort during lengthy procedures.
Provides step-by-step guidance with audio tones, allowing you to focus on the procedure without constantly watching the display.
Hands-free activation enhances procedural efficiency and flow.
Seamless setup and intuitive operation simplify even the most complex cases.
Automatically powers off if impedance peaks and the probe is not moved, preventing tissue charring.
Real-time temperature monitoring for precise control and effective ablation.

“The new Cambridge Interventional system offers an extremely handy device with excellent ultrasound visibility…[the system] is very useful for both experienced and novice users.”
Dr. Giovanni Mauri, MD
Head of the Research Laboratory on Minimally Invasive Image-Guided Treatments of the Thyroid, Unit of Diagnostic and Interventional Radiology
IRCCS Ospedale Galeazzi Sant’Ambrogio, Milan, Italy

Empower your practice with cutting-edge thyroid ablation technology. The CRF Thyroid RFA System is the future of thyroid care — schedule your demo today!
The CRF radiofrequency ablation system of Cambridge Interventional LLC (“Cambridge”) is intended for use in percutaneous, laparoscopic and intraoperative coagulation and ablation of tissue.
Read the instructions for use (“IFU”) of all medical devices prior to use. Clinical results, costs, and financial/insurance coverage may vary and are not guaranteed. The information contained in the multimedia content that is posted on the Cambridge Interventional website or that references or links to this text (“Content”) is for general informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis, or treatment; standards of medical care or training; or the instructions, indications, and contraindications for use of Cambridge Interventional devices or any other medical devices. All information is provided in good faith, however Cambridge makes no representation or warranty of any kind, express or implied regarding the accuracy, applicability, fitness, or completeness of this information; of opinions expressed; of third-party publications referenced or summarized; or of third-party services presented. Always seek the advice of your physician about a medical condition. Never disregard professional medical advice, or delay in seeking it, because of something you have read or seen in this Content.
Reported adverse events or complications for RF ablation or coagulation procedures include, but are not limited to, the following (the long-term risks of RF ablations have not been established): abscess, ARDS (acute respiratory distress syndrome), arrhythmia, ascites, atrial fibrillation, bile duct injury, bile leakage, biliary fistula, biloma, bleeding, bone degeneration, bone fracture, bronchial occlusion, bronchopleural fistula, burn, cardiac arrhythmia, cardiac ischemia, chest tube, coughing, death, delayed hemorrhage into ablated tissue, device failure, device fracture in patient, diaphragm injury, diarrhea, edema, electric shock, emphysema, fever, fistula, hematoma, hematuria, hemoglobinuria, hemoptysis, hemorrhage, hemothorax, hoarseness, hypertension, hyperthyroidism, hypoesthesia, hypotension, hypothyroidism, infection, kidney atrophy, liver failure, liver insufficiency, multiple sclerosis exacerbation, muscle burn, muscle contracture, nausea/vomiting, nerve injury, neuropathy, nodule rupture, organ damage, pain, paresthesia, perforated colon, perforation, peritonitis, pes equinus injury, pleural effusion, pneumonia, pneumothorax, renal failure, skin burn, tumor recurrence, tumor seeding, urinary fistula, urinary incontinence, urinary retention, urine leakage, vasovagal reaction, vessel injury, vocal cord palsy, voice change, wound discharge. RF ablation procedures are not recommended for pregnant patients. Potential risks to the patient and/or fetus have not been established. General clinical residual risks for surgical procedures include anesthesia reaction, bleeding, blood clots, death, infection, organ injury, pain, and necessity for more invasive surgery, including open surgery, if complications occur.