
Thyroid
Radiofrequency Ablation
- Real-time audiovisual feedback
- Automatic data capture
- Compact, sharp, steerable probe
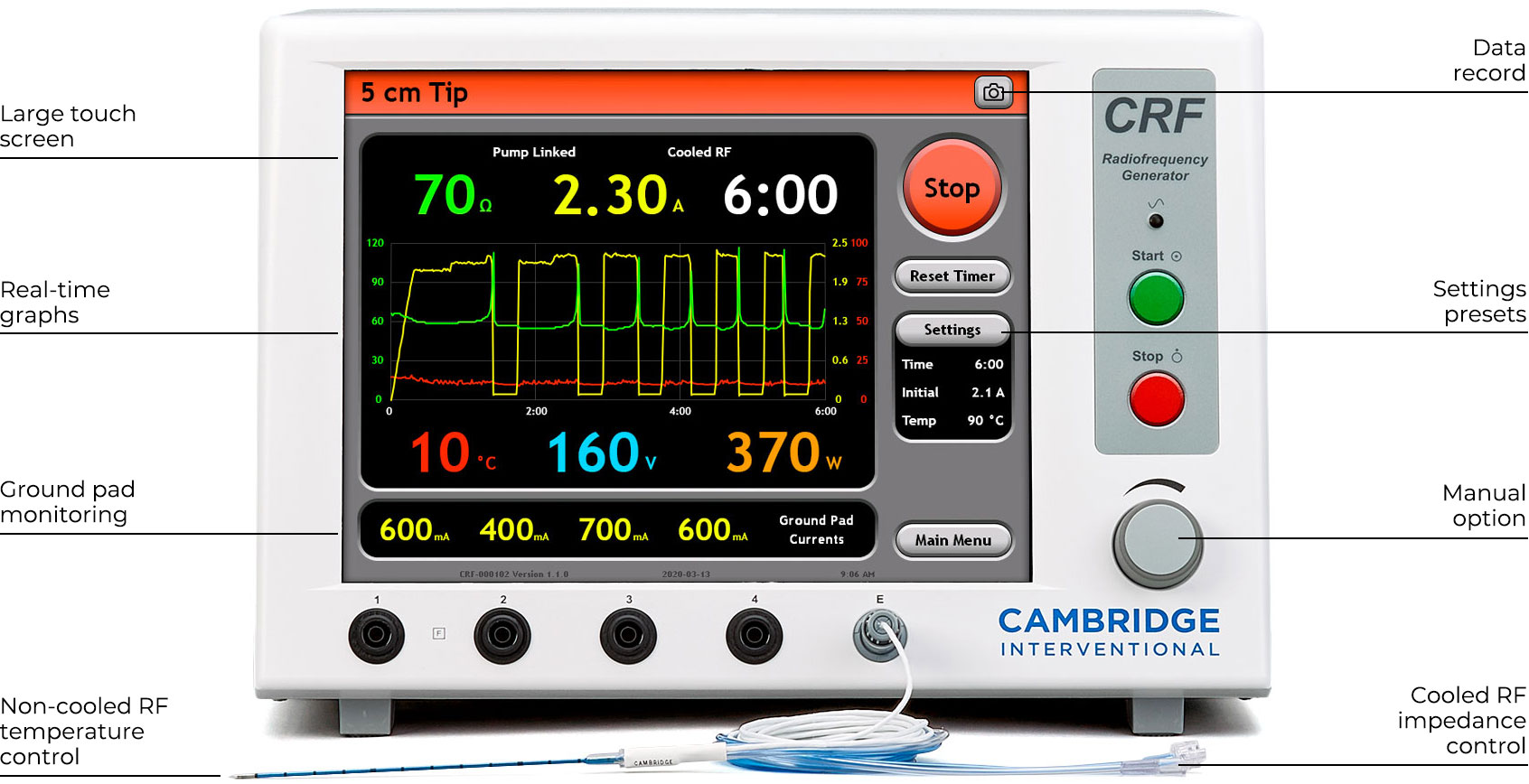
RF Generator & Pump
- Optimized to achieve large spherical ablation zones rapidly and reproducibly using a single cost- effective electrode.1
- Monitor ablation process and post-ablation heat retention with real-time graphs during and after ablation.
- Simplified operation with large color-coded touch screen, settings presets, pump synchronization, footswitch, and electronic record.
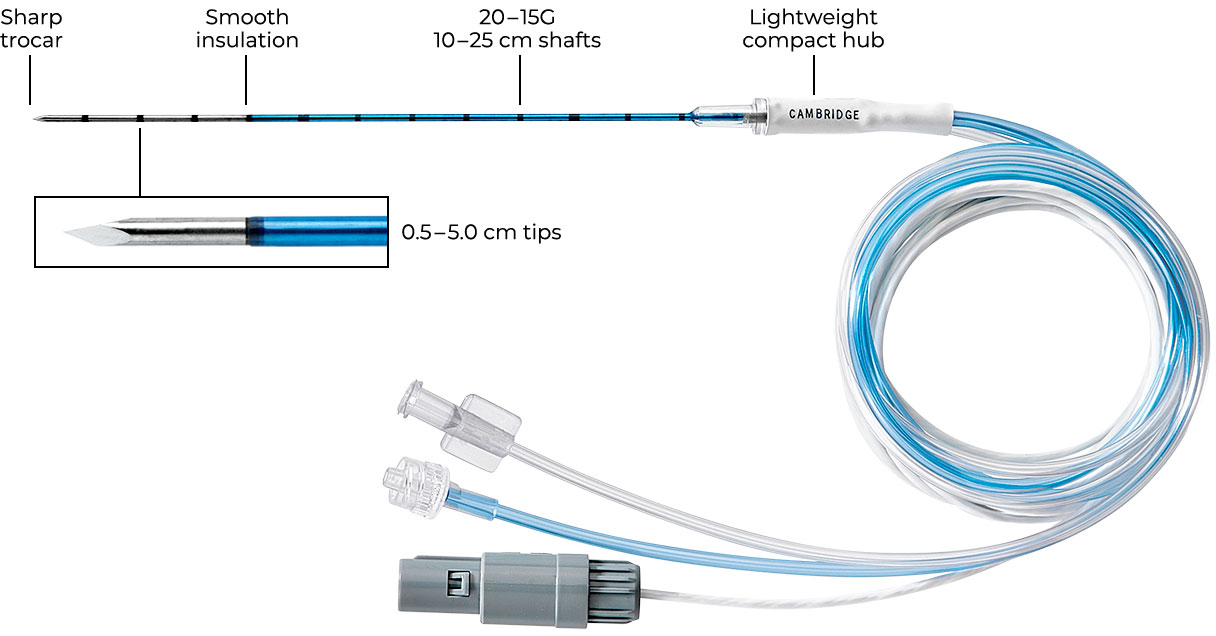
One-Piece Electrode
- Size options for many ablation applications
- Sharp tip reduces need for skin incision
- Low profile hub reduces hand fatigue and improves anatomical clearance
Two-Piece Electrode
- Coaxial design allows biopsy before ablation
- Stock inexpensive introducers with a variety of tip lengths
- Use up to three electrodes simultaneously

Ablation Sizes Diverse Applications
Large Rapid Spherical Reproducible Ablation Volumes1,2
Ben-David E, Nissenbaum I, Gurevich S, Cosman ER Jr & Goldberg SN. Optimization and characterization of a novel internally-cooled radiofrequency ablation system with optimized pulsing algorithm in an ex-vivo bovine liver. International Journal of Hyperthermia 2019; 36(2):81-88.

RFA Innovation Since 1952
The team at Cambridge Interventional has decades of experience in development, manufacturing, and sales of Class I, II, and III RFA and other medical devices. Our quality system is ISO 13485:2016 certified. The CRF radiofrequency ablation system has received FDA marketing approval, the CE mark for distribution in the EU, and other regulatory approvals.

1971 AANS: Bernard J Cosman, MS (MIT) founded Radionics® and was the first to market an RF generator in 1952. MIT Professor Eric R Cosman, Sr, PhD, developed the Cool-tip® RFA system for tumor ablation in the 1990’s.8

2007 SIS: Prof. Cosman founded Cosman Medical8 in 2004 and invented myriad RFA devices for neurosurgery and pain. His son, Eric R Cosman, Jr, PhD (MIT) founded Cambridge Interventional and developed the CRF system.
Indications for Use & References
The CRF radiofrequency ablation system is intended for use in percutaneous, laparoscopic and intraoperative coagulation and ablation of tissue. Read the Instructions for Use before use. Radiofrequency ablation is a well-established and reproducible method for performing percutaneous, minimally invasive, thermal ablation of neoplastic disease.1 Below are selected literature current at the time of this publication.
-
Shown are average ellipsoidal ablation zone sizes generated using the CRF system in bovine liver ex vivo at nominal initial temperature 20°C. Ablation size and shape may differ clinically and lead to incomplete treatment or unintended damage to nearby structures.
-
Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases in 167 patients. Eur Radiol. 2004 Dec;14(12):2261-7.
-
Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: A unified approach to the underlying principles, techniques, and diagnostic imaging guidance. AJR. 2000: 174(2): 323-31.
-
Peyser A, Applbaum Y, Khoury A, Liebergall M, Atesok K. Osteoid osteoma: CT-guided radiofrequency ablation using a water-cooled probe. Ann Surg Oncol. 2007 Feb;14(2):591-6.
-
Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001 Oct;221(1):159-66.
-
Radionics® is a registered trademark of Integra. Cool-tip® is a registered trademark of Covidien AG. Cosman Medical LLC is owned by Boston Scientific. No affiliation with, or endorsement by, these companies is implied.

Cambridge Interventional, LLC
78 Cambridge Street
Burlington, MA 01803 USA
tel +1-781-221-5300
fax +1-781-221-5846
info@cambint.com
Contact Us
"*" indicates required fields
*Required Fields. This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
