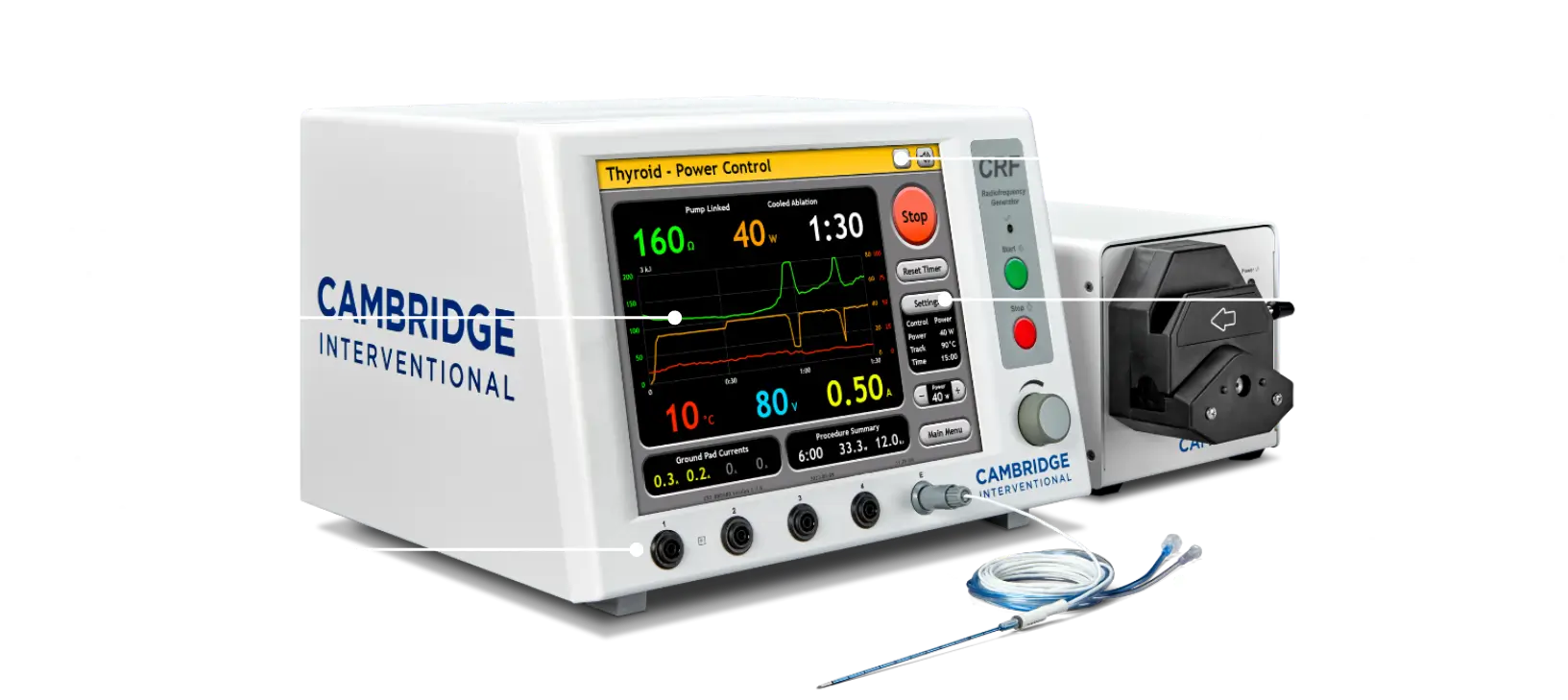
10-Year Clinical Follow-up for RFA of Thyroid Nodules
Lasting Relief: Outcomes Ten Years after Thyroid RFA Radiofrequency ablation (RFA) is a non-surgical, outpatient technique that has been used to treat benign and malignant thyroid nodules for over 20